Opthea Meets Primary Endpoint in Phase 2b Study of OPT-302 in Wet AMD
OPT-302 Combination Therapy Demonstrated Superiority in Visual Acuity over Lucentis®
| Company to Host Conference Call Today at 9:00 AM AEST 7:00PM EDT (Tuesday, August 6th U.S) -Dial-in details posted at end of release- |
- Primary outcome achieved: OPT-302 + Lucentis (ranibizumab) combination therapy demonstrated statistically significant vision benefit compared to Lucentis in wet AMD patients at 24 weeks in a trial of 366 patients
- Intravitreal OPT-302 combination therapy was well tolerated with a safety profile similar to Lucentis
MELBOURNE, Australia, Aug. 06, 2019 (GLOBE NEWSWIRE) -- Opthea Limited (ASX:OPT), a developer of novel biologic therapies for the treatment of eye diseases, today announced positive Phase 2b results demonstrating that OPT-302 combination therapy met the primary endpoint of superiority in mean visual acuity gain at 24 weeks compared to Lucentis® monotherapy in treatment-naïve patients with wet age-related macular degeneration (AMD).
The Phase 2b, randomized, double-masked, sham-controlled clinical trial recruited 366 wet AMD patients who were allocated to two intravitreal doses of OPT-302 (0.5 mg and 2.0 mg), administered monthly in combination with 0.5 mg Lucentis® over 24 weeks, versus a control group that received standard of care 0.5 mg Lucentis administered monthly.
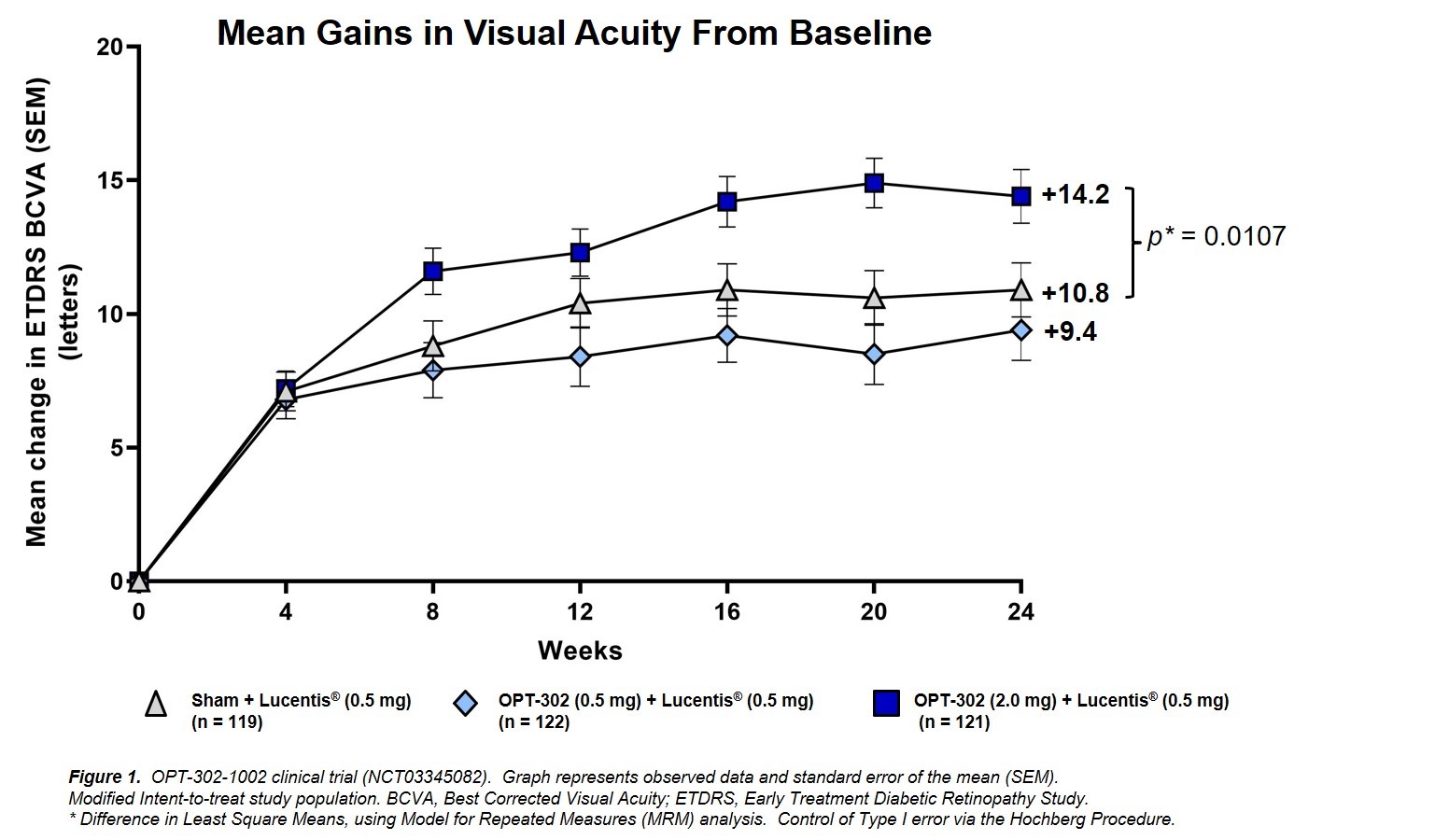
Patients administered 2.0 mg OPT-302 combination therapy gained a mean of 14.2 letters of vision from baseline on the Early Treatment of Diabetic Retinopathy Study (ETDRS) standardized eye chart at 24 weeks, compared to 10.8 letters in the control group, a statistically significant benefit of 3.4 letters (p=0.0107) (Figure 1 attached). The 0.5 mg OPT-302 low dose group had a similar outcome to the control group (+9.4 letters). Compared to Lucentis monotherapy, OPT-302 (2.0 mg) combination treatment showed improvements across multiple secondary endpoints, including a higher proportion of patients with stable vision (defined as ≤ 15 letter loss from baseline), and those gaining ≥10 and ≥15 letters of visual acuity.
“In testing for superiority against very intensive anti-VEGF-A therapy, the bar was set high. Despite this, OPT-302 (2.0 mg) combination therapy showed statistical superiority for the most accepted and sensitive primary efficacy outcome - mean visual acuity. Key secondary endpoints were very supportive of the primary outcome, and safety was favourable. Taken together, these results indicate that combined suppression of VEGF A, C and D has considerable potential as a novel treatment for wet AMD. OPT-302 may emerge as a combination treatment that can offer better vision gains than standard of care. Further registrational trials are clearly justified,” commented Professor Tim Jackson, Chief Investigator of the study, and Consultant Ophthalmic Surgeon at King’s College London.
OPT-302 intravitreal injections were well tolerated, with the safety profile similar to the control group. The independent Data and Safety Monitoring Board (DSMB) confirmed that no safety risks were identified.
Secondary endpoint results were also supportive of the primary outcome. Of those in the 2.0 mg OPT-302 combination group, 45.0% gained 15 or more letters from baseline to week 24, compared to 40.5% of patients in the Lucentis control group. The proportion of patients gaining 10 or more letters was also greater, at 70% versus 57.8%, respectively. Stable vision was achieved in 99.2% with the 2.0 mg OPT-302 combination treatment, compared to 96.7% of the Lucentis control group.
Excess retinal thickness measured on spectral domain optical coherence tomography was decreased and normalized consistently across all treatment groups by week 24. In the 2.0 mg OPT-302 combination group, mean central subfield thickness (CST) was reduced by -147 µm, from 414 µm at baseline to 266 µm at week 24. The mean CST was reduced by -134 µm, from 413 µm at baseline to 278 µm at week 24 in the Lucentis control group.
“To achieve this highly significant result and meaningful additional clinical efficacy with OPT-302 in a trial powered for superiority, against a Lucentis standard of care control arm that outperformed relative to prior published studies, is a great achievement,” commented Dr Pravin Dugel, Managing Partner of Retinal Consultants of Arizona and clinical professor at the University of Southern California Roski Eye Institute, Keck School of Medicine, and study investigator on the trial.
“OPT-302 has the potential to be a game-changer in the treatment landscape, not just for wet AMD but also for other debilitating retinal vascular diseases where there remains a significant unmet medical need for more efficacious therapies. The Phase 2b trial results demonstrate for the first time, that clinically meaningful gains in visual acuity approaching 3 lines of vision (15 letters) may be possible with OPT-302 combination therapy targeting a novel mechanism of action,” added Dr Dugel.
Opthea is in a strong cash position with ~A$20m cash and an additional ~A$14m from an anticipated Research and Development tax rebate later this year. Furthermore, the Phase 2b trial reported 6 months ahead of schedule leading to substantial cost savings for the Company. Opthea is fully funded through the remaining Phase 2b trial close-out activities and completion of the ongoing Phase 2a study in diabetic macular edema. In addition, the Company has sufficient capital to prepare for registrational Phase 3 trial activities and evaluation of all strategic and corporate options.
“We are extremely pleased with the significant positive outcomes from this study. They further support our conviction that OPT-302, the first ‘Trap’ inhibitor of vascular endothelial growth factors C and D designed specifically for the eye, can improve patient outcomes in wet AMD and other retinal vascular diseases,” commented Dr Megan Baldwin, CEO and Managing Director, Opthea Limited.
“We are delighted that the clinical results from this study support advancing OPT-302 into pivotal, registrational Phase 3 development, and firmly believe that OPT-302 will have a significant commercial role in the treatment landscape for wet AMD patients. We express our gratitude to the patients, investigators and site staff who participated in the study. Additional analyses of the Phase 2b study are ongoing, and we look forward to presenting detailed data at future ophthalmology conferences, as well as reporting topline data from our ongoing Phase 2a clinical trial of OPT-302 in patients with persistent diabetic macular edema, anticipated in early 2020.”
Conference call details:
Opthea Limited will hold a conference call to discuss the above results and welcomes participation from interested parties.
Conference ID 10001623
Australia
Wednesday 7th August, 2019
9.00 am (Australian Eastern Standard Time)
Australia Toll Free: 1800 908 299
Australia Alt. Toll Free: 1800 455 963
USA
Tuesday 6th August, 2019
7.00 pm (Eastern Daylight Time)
USA/Canada Toll Free: 1855 624 0077
United Kingdom
Wednesday 7th August, 2019
12.00 am (British Summer Time)
UK Toll Free: 0800 051 1453
About OPT-302
OPT-302 is a soluble form of vascular endothelial growth factor receptor 3 (VEGFR-3) or ‘Trap’ molecule that blocks the activity of two proteins (VEGF-C and VEGF-D) that cause blood vessels to grow and leak, processes which contribute to the pathophysiology of retinal diseases. Opthea is developing OPT-302 for use in combination with inhibitors of VEGF-A (e.g. Lucentis®/Eylea®). Combination therapy of OPT-302 and a VEGF-A inhibitor achieves more complete blockade of members of the VEGF family, blocking mechanisms contributing to sub-optimal responses to selective VEGF-A inhibitors and has the potential to improve vision outcomes by more completely inhibiting the pathways involved in disease progression.
Phase 2b Study Design
Opthea’s Phase 2b clinical trial was an international, multi-centre, prospective, sham-controlled, double-masked, superiority study that enrolled 366 treatment-naïve patients with wet AMD who were randomized in a 1:1:1 ratio to receive one of the following treatment regimens administered every 4 weeks for 24 weeks: OPT-302 (0.5 mg) in combination with ranibizumab (0.5 mg); OPT-302 (2.0 mg) in combination with ranibizumab (0.5 mg); or sham in combination with ranibizumab (0.5 mg).
Further details on the Company’s clinical trials can be found at: www.clinicaltrials.gov, Clinical trial identifiers: NCT02543229, NCT03345082 and NCT03397264.
About Wet AMD
Wet (neovascular) age-related macular degeneration, or wet AMD, is a disease characterized by the loss of vision of the middle of the visual field caused by degeneration of the central portion of the retina (the macula). Abnormal growth of blood vessels below the retina, and the leakage of fluid and protein from the vessels, causes retinal degeneration that leads to severe and rapid loss of vision. Wet AMD is the leading cause of blindness in the developed world in individuals aged over 50 years and its prevalence is increasing. Without treatment, wet AMD patients often experience a rapid decline in visual acuity.
Standard of care treatments for wet AMD and DME include the VEGF-A inhibitors Lucentis® (Roche/Novartis) and Eylea® (Regeneron/Bayer), which do not inhibit VEGF-C or VEGF-D. Sales of Lucentis® and Eylea were over $US3.7BN and $US6.2BN in 2018 respectively. Approximately half of the people receiving Lucentis®/Eylea® do not experience a significant gain in vision and/or have persistent retinal vascular leakage despite regular intravitreal injections. Combined administration of OPT-302 with a VEGF-A inhibitor, has the potential to improve visual acuity by more effective inhibition of the pathways involved in disease progression.
About Opthea Limited
Opthea (ASX:OPT) is a biologics drug developer focusing on ophthalmic disease therapies. It controls exclusive worldwide rights to a significant intellectual property portfolio around VEGF-C, VEGF-D and VEGFR-3. Opthea’s intellectual property is held within its wholly-owned subsidiary Vegenics Pty Ltd. Opthea’s product development programs are focused on developing OPT-302 for retinal diseases.
Inherent risks of Investment in Biotechnology Companies
There are a number of inherent risks associated with the development of pharmaceutical products to a marketable stage. The lengthy clinical trial process is designed to assess the safety and efficacy of a drug prior to commercialisation and a significant proportion of drugs fail one or both of these criteria. Other risks include uncertainty of patent protection and proprietary rights, whether patent applications and issued patents will offer adequate protection to enable product development, the obtaining of necessary drug regulatory authority approvals and difficulties caused by the rapid advancements in technology. Companies such as Opthea are dependent on the success of their research and development projects and on the ability to attract funding to support these activities. Investment in research and development projects cannot be assessed on the same fundamentals as trading and manufacturing enterprises. Therefore investment in companies specialising in drug development must be regarded as highly speculative. Opthea strongly recommends that professional investment advice be sought prior to such investments.
Forward-looking statements
Certain statements in this ASX announcement may contain forward-looking statements regarding Company business and the therapeutic and commercial potential of its technologies and products in development. Any statement describing Company goals, expectations, intentions or beliefs is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to certain risks and uncertainties, particularly those risks or uncertainties inherent in the process of developing technology and in the process of discovering, developing and commercialising drugs that can be proven to be safe and effective for use as human therapeutics, and in the endeavour of building a business around such products and services. Opthea undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events, or otherwise. Actual results could differ materially from those discussed in this ASX announcement.
| Company & Media Enquiries: | Join our email database to receive program updates: |
| Megan Baldwin, PhD CEO & Managing Director Opthea Limited Tel: +61 (0) 447 788 674 megan.baldwin@opthea.com Australia: Rudi Michelson Monsoon Communications Tel: +61 (0) 3 9620 3333 |
Tel: +61 (0) 3 9826 0399 info@opthea.com www.opthea.com U.S.A. & International: Jason Wong Blueprint Life Science Group Tel: +1 415 375 3340, Ext 4 Jwong@bplifescience.com |
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/6d09ba9f-2697-4b73-b688-66652faf98ca
 |
|
OPT-302-1002 clinical trial (NCT03345082). Graph represents observed data and standard error of the mean (SEM). Modified Intent-to-treat study population. BCVA, Best Corrected Visual Acuity; ETDRS, Early Treatment Diabetic Retinopathy Study. *Difference in Least Square Means, using Model for Repeated Measures (MRM) analysis. Control of Type I error via the Hochberg Procedure. |